Our Science

Built on expertise and groundbreaking research
Ceramedix is dedicated to developing transformative anti-ceramide antibodies to treat diseases driven by microvascular injury.
The company draws upon discoveries from the laboratories of Dr. Richard Kolesnick (Memorial Sloan Kettering Cancer Center) and Dr. Julia Busik (University of Oklahoma HSC, Michigan State University), internationally recognized authorities on sphingolipid signaling in stress response and Diabetic microvascular disease, respectively. Ceramedix’s drug candidates are protected by composition of matter and methods IP licensed from MSKCC and MSU.
Ceramide-rich platform (CRP) - a novel target
Our founders have identified CRP as a unique surface marker that selectively drives injured endothelial cell death. This process results in microvascular damage, the root cause of many diseases.
Unlike targets of most therapeutic approaches, CRP comprises lipid – a new type of target.
CRPs only form after injury and drive endothelial cell death via signaling
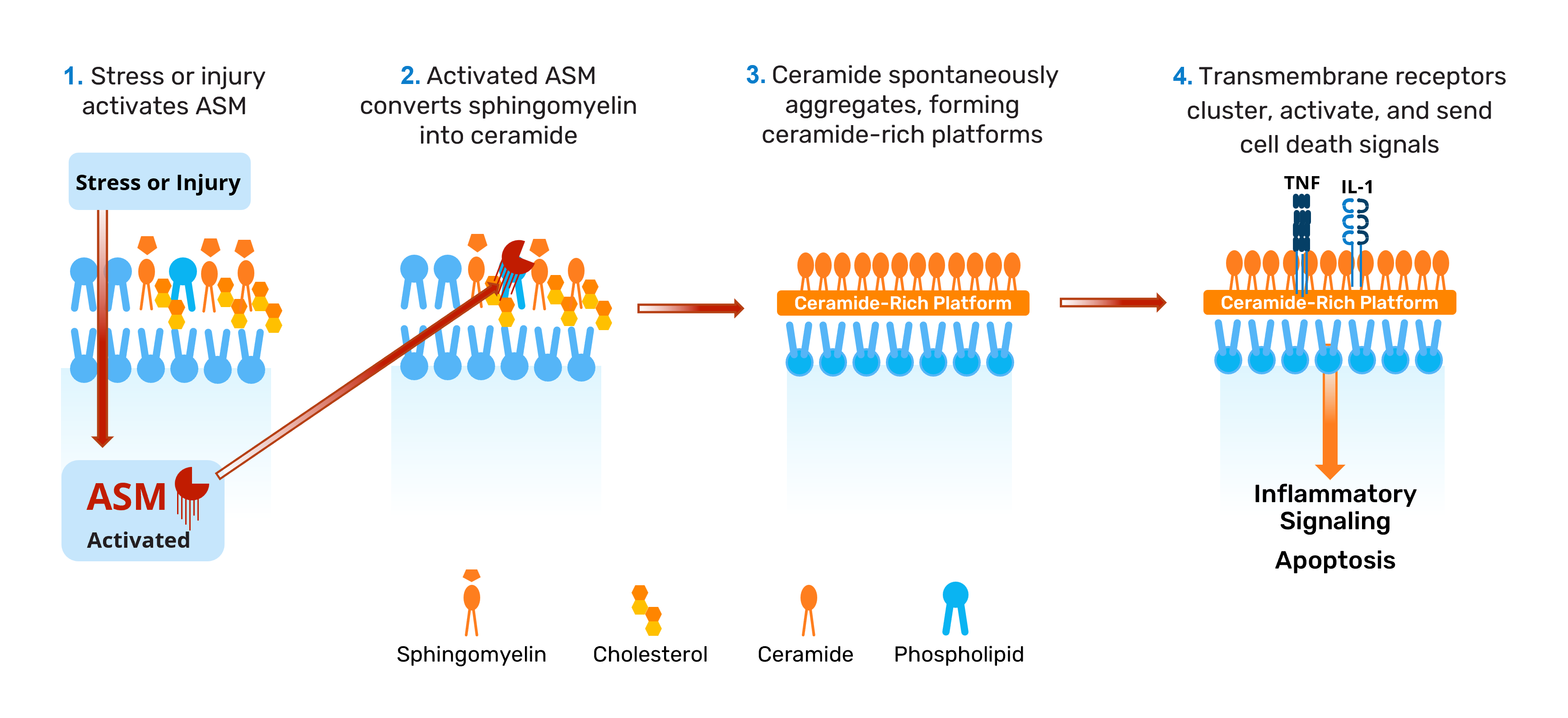
CRPs form when the lipid ceramide is generated on cell surfaces after stress or injury and spontaneously clusters into platforms that surround the cell. Ceramide is not normally a major component lipid of the exocellular leaflet of endothelial cell plasma membranes. However, following injury and stress, the enzyme acid sphingomyelinase (ASM) is activated and converts sphingomyelin – a normal membrane component – into ceramide. This process leads to formation of CRPs that serve as sites of receptor clustering and activation, amplifying cell death (apoptosis).
Endothelial cells, such as those lining our blood vessels, are especially vulnerable to CRP formation because they have the highest expression level of ASM.
CRP signaling - a key driver of injury across multiple organs
CRP-dependent apoptotic signaling has been identified as a driver of microvascular injury and subsequent organ failure across multiple organs and mammalian species:
- Retinal microvascular endothelial failure in Diabetic retinopathy in mice and humans
- Endothelial apoptosis in lung, acute lung injury and pulmonary edema
- Renal endothelial injury leading to chronic kidney and glomerular diseases
- Radiation-induced gastrointestinal tract injury and failure
Ceramedix antibodies - addressing the root cause of disease
CRP’s role in driving diverse diseases provides a strong rationale for developing a therapeutic that can block CRP formation, aiming to prevent disease progression and address critical unmet medical needs.
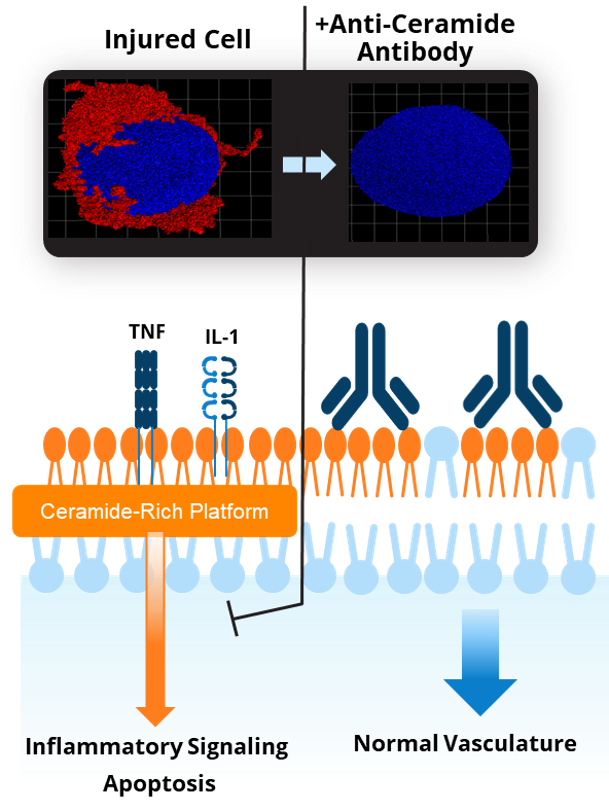
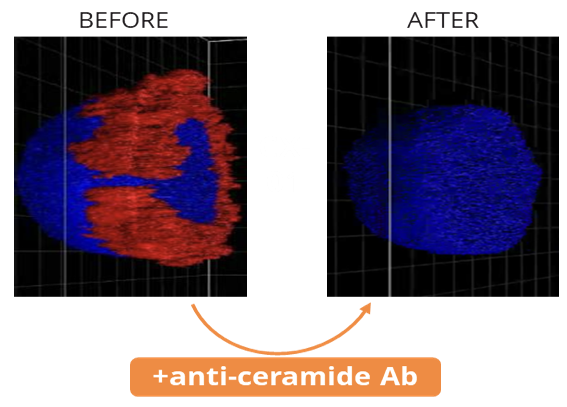
Recognizing this potential, Ceramedix has designed antibodies that target ceramide, blocking CRP formation and downstream endothelial cell death signaling. Through this mechanism, Ceramedix’s anti-ceramide antibodies address the root cause of diseases driven by microvascular injury.
In particular, our first-in-class compounds’ unique mechanism of action addresses the pathogenesis and progression of Diabetic retinopathy (DR) and Diabetic macular edema (DME). Our founders and CSO have shown that CRP formation on the surface of endothelial cells represents an initiating pathogenic event in Diabetic eye disease. Moreover, CRP formation on bone marrow-derived endothelial reparative cells renders them dysfunctional, leading to diminished retinal vascular repair in the face of Diabetes-induced damage. As a result, systemic treatment with our antibodies has the potential to not only directly stop retinal vascular damage by the action on retinal endothelial cells, but also improve vessel repair and restore homeostasis through the action on bone marrow-derived endothelial reparative cells.
Blocking CRP formation - a unique therapeutic strategy that benefits patients
Ceramedix’s approach is distinct from conventional therapies targeting symptoms rather than root cause. For instance, standard treatments for Diabetic eye diseases target VEGF elevation, which results from injury and represents the body’s attempt to heal. These treatments seek to prevent VEGF-driven wound repair that causes pathological growth of new vessels, but do not prevent injury to or enable restoration of original vessels, as is expected with our drugs.
Other approaches are also targeting non-specific inflammatory or vascular tone-related targets that are likely to have systemic toxicities, restricting their route of administration to local delivery. Our target is only formed under injury conditions, allowing for systemic administration of our drugs to treat multiple conditions, including Diabetic eye disease.
First-in-class medicine for unmet medical needs
Ceramedix aims to fulfill unmet therapeutic needs by developing first-in-class therapeutics to treat CRP-driven microvascular tissue injury, the root cause of several chronic illnesses, acute injuries, and health security threats.
We are focusing our initial efforts on DR/DME and gastrointestinal damage from high-dose radiation exposure, for which preclinical studies have demonstrated promising results.
DR/DME Program
Despite therapeutic advancements, a need persists for potentially disease-modifying drugs that prevent the progressive organ injury and failure featured in Diabetes-related complications. Novel glucose control medications have been shown to slow progression in select organs, such as kidneys. However, they do not treat the underlying organ injury that leads to eventual failure and offer little benefit to ocular disease.

To address this gap, we are developing a first-in-class DR/DME program aimed at translating a full-length anti-ceramide antibody for subcutaneous use, which is supported by promising preclinical results. Our preclinical research highlights include:
Demonstrating that systemic delivery of anti-ceramide antibodies protects against disease
Anti-ceramide antibodies delivered subcutaneously protected against DR in standardized rodent ischemia reperfusion and streptozotocin models.
Identifying CRP's key role in disease progression
Our founder and CSO demonstrated that CRP formation on the surface of endothelial cells represents an initiating pathogenic event in Diabetic eye disease. Moreover, CRP formation on bone marrow-derived endothelial reparative cells renders them dysfunctional, leading to diminished retinal vascular repair.
Confirming elevated ceramide levels in clinical samples
Vitreous samples from DR patients showed elevated levels of ceramides prone to platform formation, demonstrating clinical relevance of CRP as a target.

Radiation Countermeasures Program
With the possibility of a radiation disaster by way of nuclear detonation, accident, or radiation dispersal device, there is an urgent need for effective medical radiation countermeasures (MCMs) which must be safe and effective to treat radiation toxicities in the general population when administered 24 h after a potential nuclear disaster by way of nuclear detonation, terrorist activity or accident.
Gastrointestinal acute radiation syndrome (GI-ARS) is a major lethal radiotoxicity that causes anorexia, vomiting, diarrhea, dehydration, systemic infection, and in extreme cases, septic shock, and death within days of exposure. There are currently no FDA-approved medicines for GI-ARS, highlighting the importance of developing a new intervention.
To address the urgent need for an effective GI-ARS treatment, we are developing our anti-ceramide technology as a potential first-in-class therapy. Preclinical studies have shown encouraging results, including:
Identifying the critical role of CRP formation in disease
Our scientific founder discovered that radiation-induced CRP formation and resulting GI endothelial cell apoptosis lead to microvascular injury, driving GI-ARS development and lethality.
Verifying that anti-ceramide antibodies mitigate disease and promote survival
Studies in non-human primates and mice demonstrated that anti-ceramide antibodies effectively prevented CRP-induced endothelial cell apoptosis after irradiation. In irradiated mice, anti-ceramide antibodies mitigated GI-ARS lethality, resulting in prolonged survival with no abnormalities observed.
Research efforts to develop our product class for treating GI-ARS are strongly supported by the federal government; both in terms of direct NIAID funding, ongoing SBIR grant, and NIH/NIAID and DoD/CDMRP contract awards.
